Introduction
Blinatumomab, a bispecific T-cell antibody, has emerged as a novel therapy with a promising efficacy and tolerable safety in patients with B-cell acute lymphoblastic leukemia (B-ALL). However, there is a paucity of data on blinatumomab for patients with newly diagnosed and relapsed/refractory (R/R) B-ALL in frontline and relapsed settings outside the clinical trials. This study aimed to describe the treatment patterns, effectiveness and safety of blinatumomab in Chinese patients with B-ALL.
Methods
In this retrospective observational study, data of patients with B-ALL who received at least one dose of blinatumomab in frontline or relapsed settings between August 2021 and June 2023 were collected from electronic medical data of the Department of Haematology of the First Affiliated Hospital of Soochow, China. The primary endpoint of the study was the treatment pattern of blinatumomab therapy, including median treatment cycles. Secondary endpoints included complete remission (CR)/CR with incomplete blood cell recovery (CRi) rate, minimal residual disease (MRD) negativity (i.e., complete MRD remission, defined as MRD <0.01%), median event-free survival (EFS) and 1-year EFS rate, median overall survival (OS) and 1-year OS rate, duration of response (DOR) and adverse events (AEs).
Results
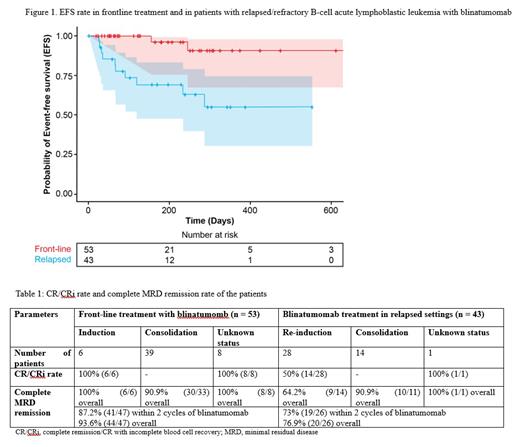
In total, 96 patients with B-ALL (53 males and 43 females) were included. Of these, 53 received frontline treatment (blinatumomab used for first line remission induction: n=6; first line consolidation: n=39; first line induction or consolidation unknown: n=8), and 43 were R/R patients (blinatumomab for remission re-induction: n=28; R/R consolidation: n=14; R/R re-induction or consolidation unknown: n=1). The median age was 34 years (range: 11-67). Among R/R patients, 18 (18.8%), 12 (12.5%) and 13 (13.5%) had received 2, 3, and ≥3 previous lines of treatments, respectively. Sixteen patients were refractory to the last treatment. Among patients using blinatumomab for consolidation, MRD status at the start of therapy was positive in 29 (30.2%), and negative in 23 (24.0%) patients. Most of the patients (66.7%) received 1 cycle of blinatumomab followed by 2 (20.8%), 3 (7.3%), 4 (4.2%), and 5 (1.0%) cycles of the drug. The median treatment cycle was 1 (range: 1-5) with a median follow-up of 205 days (range: 15-652). In frontline treatment, all those using blinatumomab for remission induction and first line unknown status achieved CR with the CR/CRi rate of 100% (14/14) after blinatumomab. In patients with evaluable MRD (n=47) in the frontline setting, 87.2% (n=41) achieved MRD negativity within the 2 cycles of blinatumomab. In the following cycles, 3 more patients achieved MRD remission with overall complete MRD remission rate of 93.6% (44/47). In R/R re-induction patients, the CR/CRi rate was 50% (14/28) after blinatumomab treatment. Complete MRD remission rate in R/R patients who achieved CR within 2 cycles of blinatumomab was 73% (19/26). Median EFS was not reached (NR) in both frontline and R/R patients and the 1-year EFS rate was 90.8% (95% CI: 67% - 97%) and 55.1% (95% CI: 30% - 74%), respectively (Figure 1). Stratification of EFS by CR status at blinatumomab initiation showed 1-year EFS rate of 93.8% and 100% in first line consolidation and remission induction patients, respectively, whereas, R/R consolidation and re-induction patients showed 1-year EFS rate of 66.8% and 59.3%, respectively. None of the frontline treated patients died until the last follow-up time. In R/R patients the median OS was not reached and the 1-year OS rate was 70.9% (95% CI: 50% - 84%). OS stratified by CR status in R/R patients showed 1-year OS of 87.5% in consolidation and 69.9% in re-induction patients. Median DOR was NR in both frontline and relapsed settings. Seven (7.3%), and 3 (3.1%) patients reported any grade, and grade ≥3 cytokine release syndrome, respectively. Immune effector cell-associated neurotoxicity syndrome was reported by 1 patient (grade ≥3). Infection of any grade and grade ≥3 was reported by 6 (6.2%) and 4 (4.2%) patients, respectively. Increase in liver enzymes and neutropenia were observed in 1 patient (grade ≥3) each.
Conclusion
This study showed that using blinatumomab for remission induction and consolidation with one to five cycles was effective in the treatment of patients with B-ALL in the frontline as well as relapsed settings and had a tolerable safety profile.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal